Application & Background
It is well known that using metals as matrices for laser desorption ionization (LDI) imaging mass spectrometry (IMS) experiments can enable the analysis of several classes of molecules that are difficult to detect with traditional organic matrices. Particularly, silver has been demonstrated to be an effective matrix for imaging many unique classes of molecules such as aromatic hydrocarbons, unsaturated fatty acids and cholesterol (1,2). However, the existing sample preparation protocols for silver deposition prior to IMS analysis has so far been limited to either using a sputtering system or spraying silver nanoparticles. While both of these techniques are highly effective for silver deposition prior to high-resolution IMS, neither has been widely utilized due to the necessity of a specialized sputtering system and difficulties in the reproducibility of nanoparticle preparation, respectively.
In this study, an innovative sample preparation protocol for silver nitrate (AgNO3) deposition for high-resolution LDI IMS was developed using the HTX TM-Sprayer. The ubiquity of automated spraying devices in laboratories performing IMS experiments and the affordability of the AgNO3 salt compound make this protocol easily adoptable for many other research groups in the IMS community.
Experimental
Sample Preparation
All animal procedures in this study were approved by the local Ethical Committee of the Université de Montréal. At necropsy, brains were removed from 12-week-old mice. For the spray protocol optimization, several mouse brains were manually cut and then sheared together in an Eppendorf tube containing metallic beads for 30 minutes. Approximately 100 μL of the homogenate was obtained using a shortened straw and immediately frozen in the straw on dry ice to form a core of tissue homogenate about 2 mm in diameter. The homogenate was then stored at -80°C. For each of the three steps of the spray protocol optimization, three 20 μm consecutive sections were cut from the homogenate using a Thermo Fisher Scientific Microm HM550 and thaw-mounted onto indium-tin oxide (ITO) microscope slides (Delta Technologies, Loveland, CO, USA) and placed in a desiccator.
For imaging experiments, mouse brains were immediately frozen on dry ice upon necropsy. The samples were then stored at -80°C. thin sections (12 μm) were cut horizontally from the brains for IMS experiments using a Thermo Fisher Scientific Microm HM550 and thaw-mounted onto indium-tin oxide (ITO) microscope slides (Delta Technologies, Loveland, CO, USA) with serial sections obtained for H&E staining.
Evaluation of Silver Salts for Method Development
Both AgNO3 and silver acetate (AgAc) were purchased from Sigma- Aldrich Canada (Oakville, ON, Canada), and solutions of each were prepared at 8.5 mg/mL in 3:1 ACN:H2O with 0.1% TFA. Each solution was manually pipetted onto the cerebellum of a mouse brain section as a 1 μL droplet. When the spectra were compared, it was found that both silver salt solutions gave similar results for intact cholesterol (CHO), but AgNO3 showed a greater number of lower mass range species, is more soluble in common organic solvents used to spray MALDI matrices and is much less expensive than other commercially available silver salts and was thus selected for further experiments (Figure 1).
Figure 1. AgNO3 and AgAc Spectra. Image was made in mMass. The spectra were normalized to the highest peak (Ag3+)
AgNO3 Spraying Protocol Optimization
Using the HTX TM-Sprayer, the parameters of AgNO3 thickness, organic solvent composition and percent of TFA added to the organic solvent were sequentially optimized to provide the highest total CHO signal and a homogenous crystal layer using the prepared mouse brain homogenate. First, the number of passes was altered to change the thickness of the deposition over six different values while keeping the other spraying parameters constant. Then, 10 different combinations of ACN, MeOH, and H2O with 0.1% TFA were evaluated at the selected optimal AgNO3 thickness while keeping the other spraying parameters constant. Then, the percentage of TFA in the solvent was varied over 4 different concentrations. Finally, 9 different combinations of varying flow rates and nozzle velocities were tested to compare the resulting crystal size from these different conditions. All tested spraying conditions are displayed in Table 1.
Figure 2. All tested spraying conditions, with final optimized conditions shows in bold.
Silver sputtering
In order to compare the silver spray protocol with an existing Ag-assisted LDI sample preparation protocol, a layer of 28 nm of Ag was deposited onto a mouse brain section using a Cressington 308R sputter coater from Ted Pella Inc. (Redding, CA, USA) at argon partial pressure of 0.02 mbar and an ac current of 80 mA, as previously described (1).
LDI IMS
All experiments were performed on a MALDI time-of-flight (TOF/TOF) ultraflXtreme mass spectrometer in positive ion mode at an acceleration voltage of +25 kV with a 2-kHz Smart Beam II Nd:Yag/355-nm laser operated using flexControl (v 3.4) and flexImaging (v 4.1) (Bruker Daltonics, Billerica, MA, USA). For the spray protocol optimization, an array of 15 x 15 pixels in the middle of the homogenate were imaged at 100 µm spatial resolution at 200 shots per pixel. For IMS of the brain sections coated via both spraying and sputtering, three brain tissue sections were imaged at 20 and 100 µm spatial resolution at 200 shots per pixel.
Data Analysis
The instrument was internally calibrated with known silver cluster peaks observed throughout the spectra using the Batch Processing function of flexAnalysis (v 3.4) for a mass accuracy of < 10 ppm. The data were then exported in imzML format and imported into R (v 3.4.4) and normalized by total ion current with the Cardinal package (v2.1). MALDIquant (v 1.19.3) was used to calculate the signal/noise (S/N) of the total CHO signals from the samples. For the spray optimization homogenate samples, the total CHO signal for each brain homogenate was calculated as the average intensity of the sum of the intensities from the two argentitated CHO signals at signals at m/z 493.2 [M + Ag107]+ and m/z 495.2 [M + Ag109]+. Pixels with S/N < 10 were thrown out and the intensities at m/z 493.2 [M + Ag107]+ and m/z 495.2 [M + Ag109]+ were extracted with the Cardinal package (v 2.1). The top and bottom 10% intense pixels were removed and the remaining signals were averaged and summed to calculate the total CHO signal used in the spray deposition optimization. Statistical significance of the effects of the thickness of the AgNO3 coating, solvent composition, and percent of added TFA was tested using multivariate ANOVA and for significant parameters, a Tukey test was then performed to test the significance between the parameter values.
AgNO3 Spraying Protocol Optimization
Figure 2. Mean total CHO signal vs. spray thickness plot used to determine the optimal thickness for AgNO3 spray coating.
As demonstrated in Figure 2, as the spray thickness of AgNO3 spray coating was increased linearly, the mean total CHO signal increased logarithmically. It was found that a spray coating thickness of 0.77 mg/mm^2 maximized CHO signal and was thus selected for use in all further experiments.
Figure 3. Crystal size and signal intensity varied based on the organic solvent composition. For all 8 solvent compositions tested, an optical image of the silver nitrate salt crystals off-tissue is shown in the top cell, and total ion current normalized IMS results of the Ag3 cluster at m/z 323.7 on the brain tissue homogenate is shown on the bottom. Scale bars represent 100 µm.
The comparison of solvent compositions revealed that there was no statistically significant difference between the total CHO signal among any of the compositions tested. Overall, a higher percent organic content yielded higher CHO signal, and solvents composed of MeOH tended to have slightly higher signal that those prepared with the same concentration of ACN. Therefore, crystal size and homogeneity of the coatings were compared. It was found that 100% MeOH + 0.1% TFA yielded the smallest crystal formation off-tissue in addition to a highly homogenous coating (Figure 3). In addition, the high organic content minimized drying time so that there was not a delay between passes that increased sample preparation time.
It was found that 0.1% TFA increased the homogeneity of the crystal coating and mean total CHO detected compared to 0% TFA, although the difference in the total CHO signal failed to reach significance, likely to do the large variability of signal observed in the homogenate sample sprayed with 0% TFA, suggesting that the resulting coating was heterogeneous. There was also no significant difference between 0.1% TFA and 1.0% and 3.0% TFA, although the total CHO signal did show a decreasing trend with higher levels of TFA (Figure 4). Higher percentages of TFA also yielded a more opaque coating, obscuring some of the histological features present in the samples. Thus, 0.1% was selected for all future experiments.
Figure 4. Effects of concentration on total cholesterol signal and signal homogeneity. For all 4 TFA concentrations tested, an optical image of the silver nitrate salt crystals off-tissue is shown in the top cell, and total ion current normalized IMS results of the Ag3 cluster at m/z 323.7 on the brain tissue homogenate is shown on the bottom. Scale bars represent 100 µm.
Finally, 9 combinations of flow rates and nozzle velocities were tested to minimize crystal size while using variable numbers of passes and track spacing to keep the total amount of AgNO3 deposited at the optimized value of 0.77 mg/mm^2. As expected, it was found that crystal size increased with increasing flow rate, as is characteristic of wetter spray conditions and decreased with increasing nozzle velocity, as is characteristic of drier spray conditions. Thus, the lowest flow rate tested was selected for optimal spraying conditions, although since there was minimal differences in the crystal coatings between the 500 mm/min nozzle velocity for 24 passes and 1000 mm/min nozzle velocity for 48, the lower nozzle velocity with less passes was selected to reduce sample preparation time. The final optimal conditions for the AgNO3 spraying are displayed in Table 1 with final optimized conditions displayed in bold type.
Olefin Visualization via MALDI IMS
Figure 5. Triplicate IMS analysis of mouse brain sections at 100 µm spatial resolution demonstrates the reproducibility of the silver spray deposition sample preparation method. The cholesterol (CHO) signal distribution from the triplicate sections are nearly identical, with greater intensity in the fiber tracts of the white matter and the midbrain. The silver deposition (Ag3+ signal) is homogeneous across the sections. Scale bars represents 1 mm.
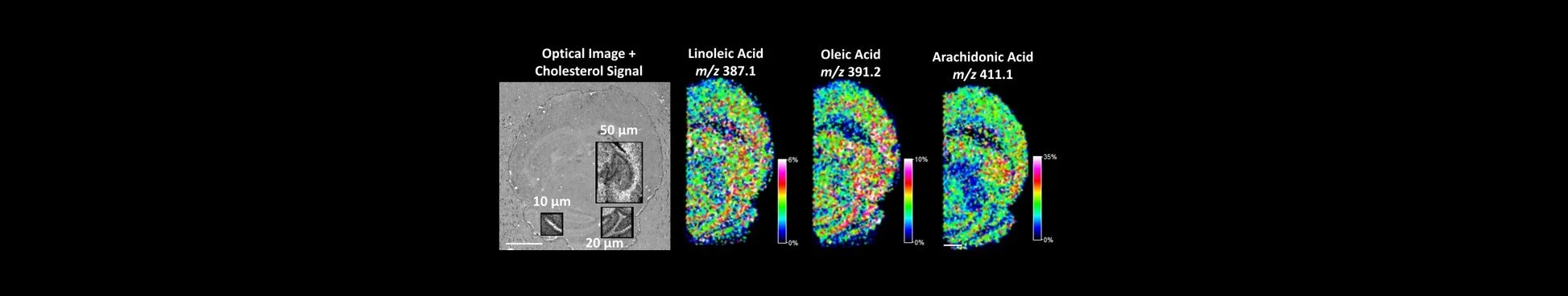
Using the optimized AgNO3 spraying technique, CHO and other olefins were visualized at both 100 µm and 20 µm spatial resolution in the brain sections. The signal for Ag3+ was also found to be stable across three technical replicates, indicating that the optimized spraying protocol is also reproducible (Figure 5). Other olefins identified in the resulting spectra included arachidonic acid, oleic acid and docohexanoic acid (Figure 6).
Figure 6. High-resolution silver- assisted IMS from a horizontal mouse brain section was performed at 50-, 20-, and 10-µm spatial resolution after spray deposition AgNO3. Above right is an optical image of the mouse brain section used with the regions that were analyzed overlayed with the IMS results of CHO in grayscale. Scale bars represent 1 mm.
Comparison with Silver Sputtering
The spectra for a sample prepared via silver sputtering versus that prepared via the AgNO3 optimized protocol were largely similar, except that the spectra from the sprayed sample contained oxidized silver species (Figure 7). This is likely attributable to the ambient conditions under which the spraying is performed compared to the vacuum system of the sputtering. In addition, sputtering does yield a more homogenous coating than spraying, allowing for higher quality high-resolution images. However, sprayed silver salt solutions are able to be removed for further section staining, while the sputtered silver coating is harder to remove for subsequent analyses.
Figure 7. Mean spectra of the cerebellum region acquired at 100 µm spatial resolution from half brain sections after AgNO3 spray deposition (top) and silver sputtering (bottom) show similar species detected.
Conclusions
The reproducibility and ubiquitous application of the silver spray protocol developed by Yang et al. makes it an attractive option for MALDI IMS of olefins. Many of these compounds play important signaling roles in neurodevelopment and neurodegeneration. The development of an accessible and adaptable AgNO3 spray protocol is important as we continue to learn more about the physiological role of these important compounds (3).
References
1) Dufresne M, Thomas A, Breault-Turcot J, Masson JF, Chaurand P. Silver-assisted laser desorption ionization for high spatial resolution imaging mass spectrometry of olefins from thin tissue sections. Anal Chem. 2013 Mar 19;85(6):3318-24. doi: 10.1021/ac3037415
2) Hinners P, O'Neill KC, Lee YJ. Revealing Individual Lifestyles through Mass Spectrometry Imaging of Chemical Compounds in Fingerprints. Sci Rep. 26;8(1):5149. Published 2018 Mar. doi: 10.1038/s41598-018-23544-7
3) Hussain G, Wang J, Rasul A, et al. Role of cholesterol and sphingolipids in brain development and neurological diseases. Lipids Health Dis. 2019;18(1):26. Published 2019 Jan 25. doi:10.1186/s12944-019-0965-z