The images and MS data presented here were provided by Dr. Ron Heeren , Florian Barré, Silvia Mas, and Anton Skriba from the M4i Institute at Maastricht University.
Application & Background
When preparing tissue sections for matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI MSI), there is often a trade-off between detection of analytes and image resolution. This is due to the fact that a wetter matrix coating can be used to promote matrix adsorption into the tissue and to lead to co-crystallization of the matrix with a greater number of molecules, thus improving signal sensitivity and strength. However, a wetter matrix deposition that uses more solvent during sample preparation can also lead to analyte delocalization within the tissue, resulting in blurred images. Here, we aimed to compare a wet spray deposition method of norhamane matrix using the HTX TM-Sprayer with a solvent-free deposition method using the HTX Sublimator to investigate the effects of these two sample preparation protocols on overall spectrum intensity and image resolution.
Experimental
Sample Preparation
Rodent brain and intestinal tissue samples were harvested by the Pharmacology Unit, School of Pharmacy, University of Camerino, Camerino, Italy, in accordance with the EC guidelines governing animal welfare and protection (EEC Council Directive 2010/63/UE), Italian legislation on animal experimentation (Decreto Legislativo n. 116, January 27, 1992) (n. 1610/2013), and had the approval of the local Ethical Committee.
Matrix Application: HTX Sublimator
90 mg of norhamane matrix was dissolved in 2 mL MeOH. The matrix mixture was pipetted onto the trough in the HTX Sublimator vacuum chamber. The trough was pre-heated to 65ºC to evaporate all of the MeOH solvent. A vacuum chamber was then drawn, and sublimation occurred at 140ºC over 180 seconds in the HTX Sublimator.
Matrix Application: HTX TM-Sprayer
Norhamane was applied to the slide at a concentration (C) of 7 mg/mL (in 2:1 v/v Chloroform: MeOH) using the HTX TM-SprayerTM. The slide was coated using the following parameters:
Table 1. Spray parameters.
MALDI Mass spectrometry imaging
Imaging was performed on a Bruker rapifleX MALDI mass spectrometer operated in negative ion mode with a 50 μm pixel spot size. All ion images were generated using FlexImaging v5.0.
Results
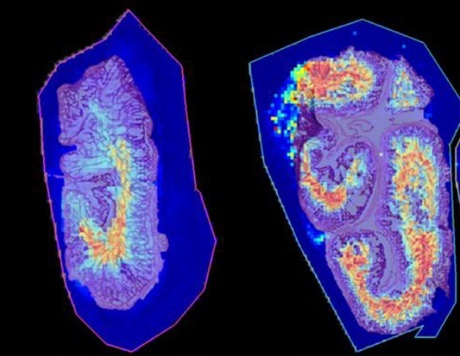
When comparing these two methods of norhamane matrix deposition for MALDI MSI of small molecules in rat brain tissue, it was found that image resolution was slightly higher in the sample prepared using the HTX Sublimator (Figure 1). In addition, there was a 10-20% increase in relative signal intensity for the top peaks of the spectrum from the tissue section prepared with the HTX Sublimator when compared to the same peaks in the spectrum from the tissue prepared with the HTX TM-Sprayer (Figure 2). We also investigated the performance of the HTX Sublimator in a MALDI MSI experiment of rat intestinal tissue to test the reproducibility of the high-resolution images acquired using the HTX Sublimator in a different tissue type (Figure 3).
Figure 1. Ion images of rat brain serial sections (12 μm) prepared using (A) the HTX TM-Sprayer and (B) the HTX Sublimator.
Figure 2. Comparison of overall spectra obtained from serial rat brain sections prepared using the HTX TM-Sprayer (blue spectra) and the HTX Sublimator (red spectra).
Conclusions
Solvent-free matrix deposition via sublimation offers several advantages over typical spraying methods. It can often improve image resolution, as no solvent is ever introduced to the surface sample to induce analyte delocalization. High-resolution images were also able to be produced in the rat intestine, which has less inherent biological structure than the brain. In addition, as we demonstrate here, signal sensitivity is maintained, or slightly improved, using sublimation for sample preparation for low mass ranges, and sublimation protocols can also be significantly faster than spraying matrix for a single slide. However, certain matrices and matrix additives (e.g. TFA) cannot be deposited via sublimation. Importantly, the results generated from each sample preparation protocol were consistent with one another, meaning these two technologies can be used in similar MALDI MSI work-flows.
Figure 3. Reconstructed ion images of bile acids in the lumen of the rat intestine. Samples were prepared using the HTX Sublimator.