All images, data, results, and conclusions were originally presented in: Seneviratne, H. K.; Hendrix, C. W.; Fuchs, E. J.; Bumpus, N. N. (2018). "MALDI Mass Spectrometry Imaging Reveals Heterogeneous Distribution of Tenofovir and Tenofovir Diphosphate in Colorectal Tissue of Subjects Receiving a Tenofovir-containing Enema."
Application & Background
Pre-exposure prophylaxis (PrEP) regimens have been demonstrated to be effective in preventing HIV infection among men who have sex with men (MSM), who are a group of individuals at high-risk for HIV infection (1). However, studies investigating adherence to various types of PrEP regimens, including pills, implants, and injections, have found a number of deterrents to compliance, such as daily pill schedules, systemic side effects, stigmatization, and fear of needles. Thus, it is of critical importance to offer at-risk individuals a wide variety of PrEP options in order to find a regimen that is effective and encourages compliance (1). Tenofovir (TFV), an HIV nucleotide analog reverse transcriptase inhibitor, has been proven to be effective when administered as a pro-drug in oral PrEP regiments and has also been investigated as a topical microbicide (2). However, studies of the effectiveness of topical TFV for HIV prevention have yielded inconclusive results (2).
In this study, we aimed to characterize the distribution of TFV and its active metabolite, TFV-diphosphate (TFV-DP) in colorectal tissue biopsies following administration of TFV-containing enemas. By using matrix-assised laser desorption/ionization mass spectrometry imaging (MALDI MSI), we were able to provide the first insight into the heterogenous distribution of TFV in mucosal tissue. Our finding that TFV and TFV-DP display differential in-tissue localization could provide insight into how drug distribution affects prophylactic activity of TFV.
Experimental
Study enrollment
All study participants were recruited under the DREAM-01 study at the Johns Hopkins University. The DREAM-01 study protocol was approved by the Johns Hopkins Medicine Institutional Review Board and conduced according to the ethical principles indicated in the Declaration of Helsinki. The study recruited HIV-negative, MSM healthy volunteers who were at least 18 years of age and had a history of receptive anal intercourse. All participants provided written consent prior to the start of the study.
Sample Collection
Four research participants were each administered two separate enemas at two different time points. One enema contained a low dose of TFV (220 mg in 125 mL = 1.76 mg/mL) and the other enema contained a high dose of TFV (660 mg in 125 mL = 5.28 mg/mL). Colorectal pinch biopsies were collected 10-20 cm from the anal sphincter using large-cup biopsy forceps and a flexible sigmoidoscope from each participants at two separate time points of 3 and 24 hours after each enema for a total of 16 biopsies.
Sample Preparation
Tissue samples were individually embedded in OCT (Sakura Finetek, Inc.) and snap-frozen in a dry ice/acetone cooling bath. Using a Leica CM3050S cryostat (Leica Biosystems), 20 μm tissue sections were cut at -20ºC and individually mounted onto glass microscope slides (Fisherbrand®, Superfrost Plus).
Matrix Deposition
CHCA was applied to the slides at a concentration (C) of 10 mg/mL (in 50:50 acetonitrile:water) using the HTX TM-Sprayer Sprayer (3). The spraying took 12 minutes per slide (25x75mm). The slides were coated using the following parameters:
Table 1. Spray parameters
Figure 1. HTX TM-Sprayer in the lab of Dr. Namandje N. Bumpus at Johns Hopkins University School of Medicine.
MALDI Mass Spectrometry Imaging
Imaging was performed on a LTQ Orbitrap XLTM (Thermo Fisher Scientific), equipped with a Fourier Transform Mass Spectrometer (FTMS) with the MALDI ion source fitted with a direct beam N2 laser (λ = 337.7 nm, laser energy = 7.8 μJ). Imaging analyses were done in positive mode to collect m/z 100-1000 Da. Mass resolution of the FTMS analyzer was 60,000. Mass spectrometry data was processed using XcaliburTM 3.0 (Thermo Fisher Scientific) and ion images were generated using ImageQuestTM 1.0.1 (Thermo Fisher Scientific).
Figure 2. LTQ Orbitrap XLTM Hybrid Ion Trap Orbitrap Mass Spectrometer (ThermoFisher ScientificTM) in the lab of Dr. Namandje N. Bumpus at Johns Hopkins University School of Medicine.
HISTOCHEMICAL ANALYSES
Hematoxylin and eosin (H&E) staining was performed on tissue sections (10 μm) adjacent to those used for MALDI MSI to visualize tissue histology. Antibodies for CD4 and CD11c cells were used to conduct immunohistochemistry studies on serial tissue sections to identify CD4 T and CD11c dendritic cells, respectively.
Results
Heterogeneous Distribution of TFV and TFV-DP in Colorectal Tissue Biopsies
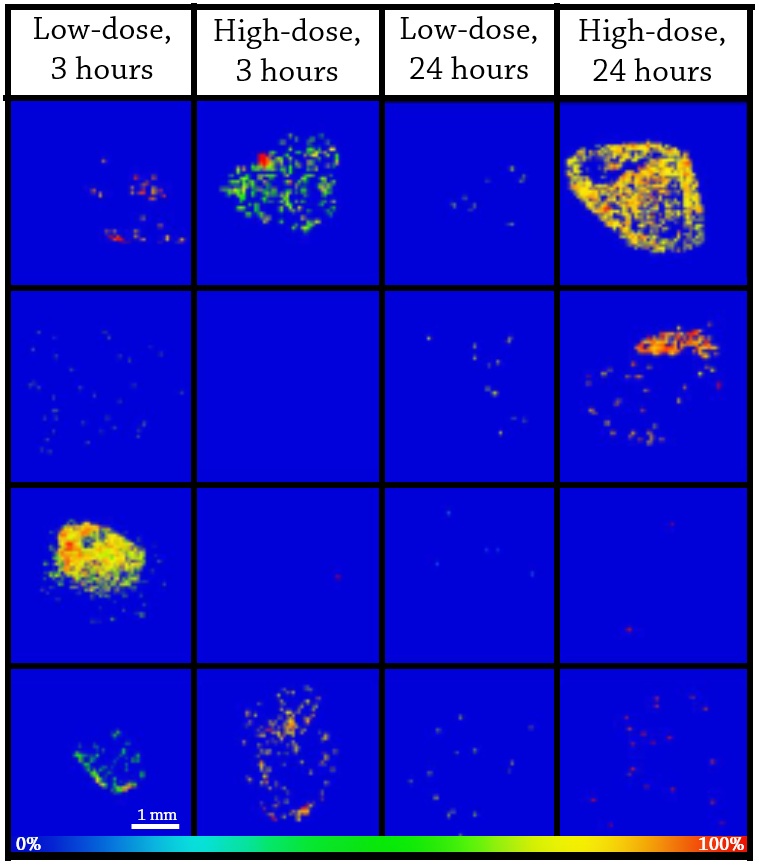
Ion images generated at 50 μm spatial resolution revealed heterogeneous distribution of TFV (m/z = 288.0860) and TFV-DP (m/z = 448.0194) in colorectal tissue biopsies following both low- and high-dose enemas (Figure 3). To verify the observed distribution pattern of TFV and TFV-DP, ion images were also generated for three phosphatidylcholine lipids that are known to be homogenously distributed in colorectal tissue (Figure 2). It was also found that the spatial distribution of TFV and TFV-DP varied with time and dose inconsistently among the four research participants. For example, only half of the research participants (J001 and J004) had detectable levels of TFV at 3 hours following the high-dose enema (Figure 4). TFV-DP (data not shown) was also variably detected in the biopsies from the four research subjects.
Figure 3. (A) H&E stain of colorectal tissue biopsy. MALDI MS ion image of TFV (m/z = 288.0860 Da) (B), TFV-DP (m/z = 448.0194 Da) (C), PC (34:1) (m/z = 760.5856 Da) (D), PC (16:0/OH) (m/z = 496.3403 Da) (E), and PC (36:2) (m/z = 786.6013 Da) (F). Spatial resolution for MALDI MS ion images was 50 μm. The highest signal intensity (100%) is represented by red and the lowest signal is represented by blue for each ion of interest.
Figure 4. MALDI MS ion images of TFV in colorectal tissue biopsies from 4 study partcipants (J001-J004) at two different doses and timepoints. Spatial resolution for MALDI MS ion images was 50 μm. The highest signal intensity (100%) is represented by red and the lowest signal is represented by blue for each ion of interest.
Results: TFV and TFV-DP Distribution is Independent of Immune Cells Involved in HIV Infection
Correlation of ion images of TFV and TFV-DP with immunohistochemical analyses on serial sections of colorectal tissue revealed that the heterogenous distribution of TFV and TFV-DP was not associated with the localization of CD4 T and CD11c dendritic cells (Figure 5).
Figure 5. Histochemical staining of CD4+ T cells (A) and (B) CD11c dendritic cells in colorectal tissue biopsy sections. Brown color indicates positive staining. MALDI MS ion image of (C) TFV (m/z = 288.0860 Da) and (D) TFV-DP (m/z = 448.0194 Da). Spatial resolution for MALDI MS ion images was 50 μm. The highest signal intensity (100%) is represented by red and the lowest signal is represented by blue for each ion of interest.
Conclusions
This study is the first to demonstrate that TFV and TFV-DP distribution varies in human colorectal tissue samples following enema dosing. Previous studies using tissue homogenates were unable to detect differential concentrations of the drug and its active metabolite in tissue regions, including regions completely lacking presence of TFV or TFV-DP. Furthermore, this research reveals that TFV and TFV-DP localization within colorectal tissue is not correlated with HIV-related immune cell populations. Future research using MALDI MSI can be used to optimize TFV dosing and in-tissue distribution for increased efficacy of this PReP option for HIV prevention.
REFERENCES
(1) Greene, G. J.; Swann, G.; Fought, A. J.; Carballo-Dieguez, A.; Hope, T. J.; Kiser, P. F.; Mustanski, B.; D'Aquila, R. T. (2017). "Preferences for Long-Acting Pre-Exposure Prophylaxis (PrEP), Daily Oral PrEP, or Condoms for HIV Prevention among U.S. Men Who Have Sex with Men." AIDS Behavior (21)5, 1336-1349.
(2) Baeten, J. and Celum, C (2013). "Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis." Annu Rev Med. (64), 219-232.